Lab Monday October 2, 2015
Do: P. 288, 6.6. Questions #1-6; You must show me the working out and check your answer.
Do: Definitions: p. 285, percentage composition, law of definite proportions
Do: Section 6.7 Empirical Formulas; Practice p. 292, #1. Answers to 6.7
Do: Section 6.7- Questions, p. 293, #1-5
Do: Section 6.9- Molecular Formulas, p. 298, #1
Do: Section 6.9 Questions, p. 1-5.
Pick a new topic to research: nt
Green chemistry (p. 119)
Garbage Gasification (p. 162)
Api -combustion reaction and haze relations, p. 192, p. 216
Mining heavy metals (p. 212)
Detox of contaminated land (p. 218)
Global Water Crisis (p. 373)
Explore an issue in solution (p. 390)
Fracking for oil and gas
Biofuel
Ms. Claire will be back in Class Monday October 12, 2015 - Go to Main campus for Lab
Tuesday Sept 16-18, 2015
Finish Sections 4.1, Practice, p. 155, #1
Review questions, p. 155, #5
Section 4.2, p. 157, #1
Review Questions, p. 161, #1,2
Section 4.4 p. 166, # 1, #2.
Review Questions, p. 169, #2, 3
Section 4.6, p. 175, #1
Section Review, p. 177, #2, 4
Blended Learning New Projects for Friday August 28, 2015
Projects for Chemical Reactions for science, technology, and society/
Chemical Spills-
Smelting of elements could be any metals- Diamond mining and extraction could include creating jewelry-
Gas and Oil extraction in Canada-
Acid Rain-
Gold extraction mining
Recycling of Toxic Elements from electronic –rare earth metals
Acidic Lakes and Rivers
Chemotherapy
Projects for Chemical Reactions for science, technology, and society/
Chemical Spills-
Pulp and Paper Mills
Smelting of cadmium; arsenic, sulfur dioxide, mercury
Scrubber systems
Rehabilitation of tailing ponds
Week of August 18, 2015
Questions to be complete and definitions:
Monday August 17, 2015
Review 2.1 : p 58, definition of ionic bond, (p. 59) formula unit, ionic compound
Tuesday August 18, 2015
Review 2.2: molecular element, diatomic (p. 61); molecular compound, covalent bond, bonding electron, bonding capacity (p. 62); lone pair, structural formula (p. 63);
Practice Questions: p. 65, #1; p. 67, # 2. Questions p. 69, #2.
Practice Questions 2.3 p. 73, #1-5 (start at #5).
Practice Questions, p. 77, #1.
Wednesday August 19, 2015
2.3 Chemical Bonding and Electronegativity
Define: electronegativity (p. 70); electronegativity difference (p. 70)
2.4 Chemical Formulas and Nomenclature
define, binary compound, polyatomic ionic compound, oxyanion and zero-sum rule (p. 74):
Do Practice Questions, p. 75, #1 and 2.
Do Practice Question, p. 77, #1
Do Practice Question, p. 78, #2
Naming Molecular Compounds, p. 80, Do Practice Questions, #1,2.
Review Questions Section 2.4, #1abc, 2, 3abcd, 4ab, 5, 6ab, 7abc.
Thursday August 20, 2015
Review Chapter 2: P. 88, #12-21, 33 a, b. 36 a,b,c, d. 39, 40 ab.
1 and 2nd Week of Chemistry 11-July 27- Aug 7
Homework Chemistry 11 Review:
Assessment #1 Review
1. Define matter (pr .8); empirical knowledge, theoretical knowledge, theory (p.9); IUPAC (p. 10) ; atom (p.11); Dalton’s Theory (p. 11); Bohr-Rutherford diagrams (p. 14); Atomic Number (Z)
A mass number(both protons and
neutrons)
X (element)
Z atomic number (number of protons)
2. Review Questions 1.2, Questions, p.16, #6
1.3 Ions and the Octet Rule
Define full or stable octet (p. 17); octet rule (p.17); ion (p.17)
Draw a cation (p. 18) ; valence (p. 18); anion (p. 18); Explain what a multivalent is (p. 19). Define what a polyatomic ion is (p. 20)
1.5 The Periodic Table and Periodic Law
Define what the general trends in the periodic are (p.30)
Define the following:
Group (p.31)
Period (p. 31)
Periodic Law (p. 31)
Lewis Symbol (p. 32)
1.7 Periodic Trends in Atomic Properties
Atomic radius (p. 36)
Effective nuclear charge (p. 37)
Ionic Radius (p. 37)
Ionization Energy (p. 38)
Electron affinity (p. 40)
Blended Learning Grade 11 July 2015
Start Research on the following: Use G-mail, PowerPoint or G-mail Slides
Chemistry Due on August 21
Choose from the following topics or choose another one:
Research and show the science, technology and social impacts of the following:
1. fertilizers, pesticides, household cleaning product, materials used in batteries and electronics
2. use of oil paints compared to latex paints
3. flushing pharmaceuticals (such as birth control pills down the drain and the impact on wildlife)
4. addiditves in foods (evaluate the risks and benefits to humans)
5. cosmetics and perfumes
6. cleaning products such as mildew remover
Remember to include:
Science- such as showing the science part of the project such as the chemical composition
Technology- how we use the products such as the machines
Society - how humans use the products for good purposes or not so good purposes
Exam: Chemistry 11 Thursday June 12, 2014 at 1:00 to 3:30 pm in KS 7.26
Monday May 26, 2014
IN class- Finish Gas Laws Assessment Sheet
Tuesday May 27, 2014
Shortened Periods
P. 1 8-905 am
P. 2 9:05 to 10:10 am
P. 3 10.10- 11.15 am
11.15 to 12.15 am - CPU Graduate LT 1 (Third Floor)
P. 4 12.15 to 1:20 pm
P.5. 1.20 to 2.25 '
P. 6. 2.25 to 3.30
Wednesday May 28, 2014
Finish Sections and Review
Thursday May 29, 2014
Solve Calculation Questions from textbook:
Friday May 30, 2014
Assessment on Moles in Solution Calculations
Shortened Periods
P.1 8.00 - 9:05 am
P. 2 9.05 - 10:10 am
P. 3 10.10-11.15 am
Mentorship meeting 11.15 to 12.15 pm (Go to mentorship rooms)
P.4 12.15 - 1.20 pm
P.5 1.20 - 2.25 pm
P.6 2.25 - 3.30 pm
Exam Revision
Exam Revision Practice Exams
Monday May 19, 2014- In class
Thursday May 15, 2014
Finish Lab Experiments from "Not Acids are Created Equal"
Friday May 16, 2014
Course Evaluations: 10:30- 10:50 am- EA 5- You only have to complete this
task on Friday
Wednesday May 14, 2014
Lab: Complete a table comparing the various acids from the last experiment. Answer the questions on the lab.
Monday May 5, 2014
Lab- 10 B, Vinegar
Tuesday May 6th, 2014 - See the rxns
Monday May 5, 2014
Lab- 10 B, Vinegar
Tuesday May 6, 2014
Presentations and finish Solutions and Vinegar
Wednesday May 7, 2014
Presentations and finish Solutions and Chapter 10
Thursday May 8, 2014
Calculations from Chapter 10
Friday May 9, 2014
Mentor ship meeting
Solving Moles Problems
Section 6.6 : The Composition of Unknown Compounds
Do: P. 288, 6.6. Questions #1-6; You must show me the working out and check your answer.
Do: Definitions: p. 285, percentage composition, law of definite proportions
Do: Section 6.7 Empirical Formulas; Practice p. 292, #1. Answers to 6.7
Do: Section 6.7- Questions, p. 293, #1-5
Do: Section 6.9- Molecular Formulas, p. 298, #1
Do: Section 6.9 Questions, p. 1-5.
Chapter 7: Stoichiometry in Chemical Reaction (combining moles and chemical reaction)
7.1 Mole Ratios in Chemical Equations;
Do: Solving mole ratio problems, p. 319, #1-3
Do: 7.1 Questions, p. 320, #1-5. Answers to 7.1
7.2 Mass Relationships in chemical Equations,
Do: p. 321, definition of stoichiometry, p. 323, #1-3
Do: Questions 7.2, p. 1-5.
7.3. Which Reagent Runs out First? (limiting reagent)
Do: Definitions of: limiting reagent; excess reagent ( p. 3260)
Do: Questions p.330, #1-3
7.4 Calculations Involving Limiting Reagents,
Do: p. 332, Practice, #1-3
Do : P. 334, Practice, #1 and 2.
Do: 7.4 Questions, #1-4
7.5 Actual/ Theoretical and Percentage Yield
Do Practice Question, p.338, #1and 2
Do Questions P.338, #1-5, 5,6,7
End of Chapter Review
6.4 Molar Mass
p. 275, Practice #1abc
Questions 6.4, #1-4
Practice #1abc, 2abc.
6.5 Mass and Number of Entities
P.280, Practice #1-3
P.282, Practice #1 and 2
6.5 Questions, p.283, #1-5
6.6 The Composition of Unknown Compounds
Definitions Percentage Composition, P. 284
Practice, p. 286, #1 and 2.
Practice, p. 287, #3
6.6 Questions, p. 288, # 1-5.
6.7 Empirical Formulas
Practice, p.292, #1
Questions, p. 293, #1-3
6.9 Molecular Formulas
Do: Molecular Formulas: Practice, p.298, #1
Do: Questions, p. 298, #1-5
Chapter 7.1 Mole Rati
IN Room 4.9 at CPU building for class on Monday March 24, 2014
DUE DATES: Friday Feb 21, 2014
Finish Blended learning research presentation you must finish by Monday Feb 24,2014
Finish lab report: either hand written or typed your choice try to answer as many discussion questions as possible.
Finish Worksheets for naming: 2.1- 2.5
Blended Learning Assignment Due Mar 12, 2014
Work oan presentations for Quantities in Chemical Reactions
1. Medical Malpractice because of giving patients too much or too little medication ( how often it occurs and why)
2. Chemotheraphy (medical doses and how this chemical compound treats patients)
3. Chemical production plants- how they affect soil, air and water supplies.
4. Agricultural run off in rural places (such as "pigmy elephants" and other wildlife).
5. Hormones in the waterway system ( birth control and other hormones that are feminizing fishes)
6. Plastic containers in the waterways (Garbage patch in Pacific ocean)
7. Radiation therapy ( how it works, the dosage amounts and how often you are given the treatment).
Monday Feb 10, 2014
Lab: Crystalline Structures, Aluminium Foil, Sodium (Natural element)
Lab: Single Displacement Reactions 1
Report Format for Single Displacement Reactions
Tuesday Feb 11, 2014
Review and Presentations
Wednesday Feb 12, 2014
Assessment on Periodic Table
Thursday Feb 13, 2014
Presentation for Model Building: Homework p. 67, Mini Investigation.
See Lab Modelling:2 D and 3 D models of compounds
Friday Feb 14, 2014
Blended Learning; Presentation on Chemical Compounds in science, environment, and technology.
Research, the properties of a commonly used but potentially harmful chemical substance
fertilizer,
pesticide,
household cleaning product
materials used in
electronics and batteries
1. Science Section: Show how the above substances that substance affects the environment, and propose ways tolessen the harmfulness of the substance (e.g., by reducing the amount used, by modifying one of its chemical components) or identify alternative substances that could be used for the same purpose.
For example: (Environment Section) Many commercial household cleaning products contain corrosive substances that can accumulate in the environment. There are now many “green” cleaners that do not contain these substances, although some of these products may not be as environmentally friendly as claimed.
Sample questions: Why is it more environmentally friendly to use latex rather than oil-based paint? Why should paint never be poured down a drain? What properties of some common pharmaceuticals allow them to stay in water systems and influence the growth and development of organisms? What are some ways in which this impact can be reduced?
2. (Society Section) Evaluate the risks and benefits to human health of some commonly used chemical substances (e.g., chemical additives in foods; pharmaceuticals; cosmetics and perfumes; household cleaning products)
Sample issue: Artificial sweeteners, such as aspartame, are used as sugar substitutes to reduce calories in processed foods and beverages. How can the use of non-stick cookware help reduce the amount of fat in our diet? What risks are associated with the use of such cookware? What are the risks and benefits of using sunscreens that contain PABA? What are the risks and benefits of using insect repellents that contain DEET?
Unit #1 Unit Revision Sheet
1. Who proposed the atomic theory of matter in 1809?
2. Name the particles of the atom and what are their relative sizes?
3. What numbers are important for organizing the periodic table?
4. Draw an outline of the trends of the period Table.
5. Name nonmetallic groups of elements.
6. Elements in the same chemical family (meaning same columns) have the same number of electrons.
7. Draw the element atomic diagram using the Bohr- Ruther ford and Lewis for the following elements:
|
Element Symbol |
Name |
Bohr-Rutherford Diagram
|
Lewis Diagram |
|
C |
|
|
|
|
|
Nitrogen |
|
|
|
He |
|
|
|
|
|
Sodium |
|
|
|
Li |
|
|
|
|
Mg |
|
|
|
|
K |
|
|
|
8. Fill out the table below:
|
Element |
Protons |
Neutrons |
Electrons |
|
Carbon
|
|
|
|
|
Hydrogen
|
|
|
|
|
Lithium
|
|
|
|
|
Sulfur
|
|
|
|
|
Chlorine
|
|
|
|
9. In history is the current periodic table configuration the only periodic table. Show a picture to help you explain if this helps.
Chemistry Thursday January 16, 2014
Start of course: Introduction (name tag), Book (where to buy), Outline-give out to students.
Chemistry Monday January 21, 2014
G-mail and Gizmos- Project
Tuesday January 22, 2014
Start with Definitions from first section- Sheets
Wednesday January 23, 2014
Calculation Questions from Textbtook
Thursday January 24, 2014
Friday January 25, 2014
Exam revision
Gases
Kinetic theory of gases (elastic and legible)
Gay Lussac- when temperature increases what happens to the pressure inside of the container
Solutions
Definition- solvent, solute, and solution
homogeneous, and heterogeneous
Quantities in Chemical Equations
Mole value
Mole ratio
Balancing equations
Calculation from Mol per liter given moles
Chemical Equations
Double Displacement, Single Displacement, synthesis, combination and neutralization reactions. Combustion and incomplete combustion.
Chemical Bonding
Chemical Naming
Electronegative
Strong bonds, result in different structures
Periodic Table
Columns, rows, periodic law, determine the number of protons, neutrons and electrons
Bohr -Rutherford model
Lewis Diagrams and bonding
Know how to cross the charges to create a compound
Exam Revision Equationsand Science and technology questions
Week of Monday Oct 28- Nov 1
Monday- Review of Calculation of Moles
Tuesday- Finish Limiting Reagents; Start Solute, Solvent, Solutions and Examples and Lab 9A and 9B examples.
Wednesday- Finish; Chapter 8 Definitions
Thursday-Shortened Periods
PERIOD 1 - 8.00 - 9.05 AM
PERIOD 2 - 9.05 - 10.10 AM
PERIOD 3 - 10.10 - 11.15 AM
CPU POTENTIAL GRADUATES MEETING
(11:15AM – 12:15PM)
PERIOD 4 – 12.15 - 1.20 PM
PERIOD 5 - 1.20 - 2.25 PM
Friday- Blended Learning Assignment for Calculations to be handed in after the break.
Week of Monday Oct 21-25
Finish Presentations -for Bio- Remediation
Tuesday- Presentations
Wednesday- Finish Calculations (6.7-6.9; 7.1-7.3)
Thursday- Finish Calculations below continue above
Friday-Lab on Dilutions and Calculations
Week of Monday Oct 14(blended learning )
Tuesday -holiday
Wednesday- Presentations
thursday- Presentations
Friday- Lab 9B Mystery Solutions
Week of Monday Oct 7, 2013
Blended Learning Day: Class -
Work on Project for Oct 8- using either G-mail or using PowerPointsTuesday Oct 8, 2013
Solving Moles Problems
Section 6.6 : The Composition of Unknown Compounds
Do: P. 288, 6.6. Questions #1-6; You must show me the working out and check your answer.
Do: Definitions: p. 285, percentage composition, law of definite proportions
Do: Section 6.7 Empirical Formulas; Practice p. 292, #1. Answers to 6.7
Do: Section 6.7- Questions, p. 293, #1-5
Do: Section 6.9- Molecular Formulas, p. 298, #1
Do: Section 6.9 Questions, p. 1-5.
Chapter 7: Stoichiometry in Chemical Reaction (combining moles and chemical reaction)
7.1 Mole Ratios in Chemical Equations;
Do: Solving mole ratio problems, p. 319, #1-3
Do: 7.1 Questions, p. 320, #1-5. Answers to 7.1
7.2 Mass Relationships in chemical Equations,
Do: p. 321, definition of stoichiometry, p. 323, #1-3
Do: Questions 7.2, p. 1-5.
7.3. Which Reagent Runs out First? (limiting reagent)
Do: Definitions of: limiting reagent; excess reagent ( p. 3260)
Do: Questions p.330, #1-3
7.4 Calculations Involving Limiting Reagents,
Do: p. 332, Practice, #1-3
Do : P. 334, Practice, #1 and 2.
Do: 7.4 Questions, #1-4
Thursday Oct 9, 2013
Short Quiz- Based on Pre Quiz of Molar Mass, moles, Avogadro's number and questions from textbook.
Friday Oct 10, 2013
Lab on Solutions 9A-
Week of Monday Sept 30, 2013
Monday- Review for Assessment
Tuesday- 6.3-Avogadro's number, moles conversion to grams. (p. 268)
Definition (p. 268) Avogadro's constant (number) and mole
amount (n) -
Avogadro's contant - 6.02 x 1023
Entities- assume if not
molecules, atoms, formula units
Molar Masses (p. 275)
n (moles) = mass (m ) ___
molar mass (M)
N = n NA
N = (number of entities)
n= (number of moles)
NA = Avogardro's number ( 6.02 x 1023 )
Practice Questions:p. 282, # 1-3 (answers given in textbook)
Section 6.5 - #1- 4 - Avogadro's number, moles and entities
Section 6.6-
Percentage Composition (p. 284) definition
Law of Definite Proportions- ( p. 28) Think of Lab - nuts and bolts and Hydrate Lab- from dark blue to light blue.
Wednesday- Assessment on Chemical Reactions
Thursday- Review moles problem and the mole box
Friday Lab:Hydrate Lab using moles conversion.
Week of Monday Sept 23, 2013 Review for Assessment
Assessment: Chemical Reactions
Definitions (p. 152)
Chemcial Reaction
Law of conservation of mass
Balancing equations and types of Chemical reactions
Synthesis Reaction (p. 156)
Decomposition Reaction (p. 159)
Molecular compounds( p. 61)
Ionic compounds ( p. 59)
Formula unit (p.59)
Single Displacement Rxn (p. 164)
Double Displacement Rxn (p. 173)
Solubility (p. 173) using the Solubility Table
Tuesday- Review Balance Equations
Wednesday- Start of Mole and Molar Mass and Avogadro's Numbers
Thursday- Sulfur Dioxide, Carbon Dioxide, and Nitrogen Dioxide. Science, Society and Technology. See activity on Pollution and Acid Rain
Also full link:
https://docs.google.com/presentation/d/1P20nB9HVUWbUtO3RjAs98dXD2EVXburzmX8kBeepumQ/edit?usp=sharing
Friday- Lab Hydrate and pH level.
Week of Sept 17, 2013
Assessment: Finish Lab Report for Double Displacement Rxn Report
Tuesday- Balancing Equations
Wednesday- Balancing Equation and Chemical Rxns
Thursday- Balancing Equation- and Lab 4E Copper and Copper Lab- experiment Questions
Friday- Lab Neutralization Reactions Lab 4C
Week of Sept 9, 2013
Assessment: Science, Technology Society of various substances
Tuesday Sept 10, 2013
Wednesday Sept 11, 2013 Presentations
Thursday Sept 13, 2013
Friday- Lab 4E Lab- in class.
Week of Monday Sept 2, 2013
Assessment: Bonding and Naming Wednesday
Tuesday Sept 3- Review for bonding and naming
Wednesday Sept 4- Assessment
Thursday Sept 5- General Chemical Equations
Friday Sept 6- PD Day- No classes
Week of Monday August 26, 2013
Monday August 26, 2013- Review of Compounds, elements and atoms and naming.
Tuesday August 27, 2013- Finish Compounds and naming of ionic compounds for double displacement questions.
Wednesday August 28, 2013- Create compounds, using model molecules, and also jig saw puzzle
Thursday August 29, 2013- Finish Covalent compounds or displacement reaction p.108, #1-5, 4ab, 5ab,
p.65, #1a-e; p.69, #1-2 a-e
Friday August 30, 2013- Chemistry Lab 4. One Flight of stairs.
Week of August 19, 2013
Monday August 19, 2013- Work on Review Sheet for Assessment
Tuesday August 20, 2013- Quiz- Assessment for Speed, Diagram,Scale Diagrams. Page References from textbook, p.14, 15, 17, 18, 19, 30, and 31
Wednesday August 21, 2013- Hand in Graphs and Questions from sheets: Ionization, Atomic Radius, Electron Affinity,
Thursday August 22, 2013- Naming Compounds
Friday August 23, 2013- Lab in EA2- CPU building- No chem lab for A level practice examination.
Study Notes for Exam

|
24731613-Chemistry-Study-Guide-Notes-For-Final-Exam-SCH3U-Grade-11.pdf Size : 284.585 Kb Type : pdf |
Practice Exams
Practice Exam #1

|
chemistry practice final exam june 2012.pdf Size : 388.015 Kb Type : pdf |
Practice Exam #2

|
Practice+Exam.pdf Size : 162.935 Kb Type : pdf |
Monday May 20, 2013
Lab Questions: Finish 9-B-Questions
Lab Questions: Finish Vinegar Questions...
Tuesday to Thursday May 30, 2013
Quiz on May 31, 2013
Gizmos for Boyle's and Charles' Law
Last Calculation Set for Gases:
11. 7 Atmospheric Pressure conversions- P. 543, #1 .
11.7 Questions, p. 546, # 1-7- In a group choose to a few to do and put together.
11.8: The Gas laws- Absolute Temperature and Charles Law p. 549, # 1 and 2. P. 553, #1-9. In a group choose to do a question.
11.9: The Gas Laws- Boyle;s Law Gay Lussac’s Law and the Combined Gas Law, p. 559, # 1- 3. Questions, p. 562, # 1- 11.
12.1 Avogadro’s Law and Molar Volume, p. 579, #1-2. P. 581, #1-3
12.2. Ideal Gasses and the Ideal Gas Law, p. 587, #1-2. Questions, p. 589, #1-4.
12.4, Gas Mixture and the Law of Partial Pressures, p. 594, #1- 2. Page 597, Questions, #1-4.
12.5 Reactions of Gases and Gas Stoichiometry, p. 599, #1
Hand in- Friday May 31, 2013.
Please show all of your working on an A3 or A4 Sheet. Organized by Sections (11.1 and so on)
Quiz (Assessment) on Charles, Boyles on Wednesay May 29, 2013
· Pressure conversion ( 1 Question)
· Charles Law (1 Question)
· Boyles’ Law (1 Question)
· Gay Lussac ( 1 Question)
· Combined Gas Law ( 1 Question)
· Ideal Gas Law ( 1 Question)
This quiz will be a calculation assessment. Please make sure you finish all of the questions before the quiz.
Tuesday May 21,2013
Quiz- on Lab Questions...why must you ensure that the beaker and buret are clean...what is the difference between volumetric and graduated beakers/pipettes.
Dilution- how do you conduct a dilution. you may use an example in class.
Solution- how do you create a Molar Solution using hydrates.
Definition- solution, solutes, solvent, amount concentration, dilution. Immiscible and miscible.
Wednesday and Thursday May 22 and 23, 2013
Presentation on Air Quality for Science, Technology and Society
Friday May 24, 2013
NO CLASS PUblic Holiday
Tuesday May 8, 2013
Solubility 9.5 - calculate the Stoichiometry Questions. Balancing Equations with moles. Activity for Class calculation questions.
Wednesday May 9, 2013
Start on: Charles Law,
Thursday May 10, 2013
Friday May 17, 2013
Lab Neutralization Reaction-4B
Monday may 6, 2013
Finish lab calculations hand in limiting reagents questions.
Tuesday May 7, 2013
Finish concentration questions from Lab (Copper Sulphate, and dilution problem set).
Section 8.7 Finish p. 405, # 3-4. Dilution problems.
Section 8.8, Finish p. 408, # 3-4. Concentration of consumer products, such as volume concentration. Section 8.8, Questions p. 411, # 1- 4.
Wednesday may 8, 2013
Start with and Review for Lab on Thursday.
Solubility and Solutions Review
Thursday may 9, 2013
Start with gases.
In class electricity. Shortened Periods. In ECA room.
PERIOD 1 - 8.00 - 9.05 AM
PERIOD 2 - 9.05 - 10.10 AM
PERIOD 3 - 10.10 - 11.15 AM
PERIOD 4 - 11.15 - 12.20 PM
Mentorship Meeting (12.20 –1.20 PM)
PERRIOD 5 - 1.20 - 2.25 PM
PERIOD 6 - 2.25 - 3.30 PM
PERIOD 7 – 3.30- 4.45 PM
Friday may 10, 2013
Lab titration- lab
Monday April 29, 2013
Please put your observations on Friday into this Google Doc
Please finish the "Limiting Reactant Assessment"
Tuesday April 30, 2013
Finish Solute, Solvent and calculation Questions and Calculation of Concentration Questions
Wednesday May 1, 2013 NO SCHOOL HOLIDAY
Thursday May 2, 2013
Continue on Chapter Definition of Solutions and Calculation % Questions. 8.6 to 8.8 Calculations
Here is the slide for Today's Class
Friday May 3, 2013
Lab main campus- 9B Qualitative and Dilution Experiment
Please finish the presentations below to Present on Tuesday April, 23, 2012 - MUST BE FINISHED
Work on presentations for Quantities in Chemical Reactions
1. Medical Malpractice because of giving patients too much or too little medication ( how often it occurs and why) Shaheen
2. Chemotheraphy (medical doses and how this chemical compound treats patients) (Fitri choice)
3. Chemical production plants- how they affect soil, air and water supplies. (Jeremy)
4. Agricultural run off in rural places (such as pigmy pigs and other wildlife) (Izzat)
5. Hormones in the waterway system ( birth control and other hormones that are feminizing fishes) (Audrey)
6. Plastic containers in the waterways (Garbage patch in Pacific ocean) (Fitri choice)
7. Radiation therapy ( how it works, the dosage amounts and how often you are given the treatment). (Khalil)
Tuesday April 23, 2013
Start on Calculation Questions for Limiting Reagents
Percentage Yield Questions: p. 338, #1 a and b.
Wednesday April 24, 2013
8.2 Solutions and their characteristics, heterogeneous mixture, concentration ,
alloy and other mixtures,
Aqueous Solution.
Amalgam- Do Worksheet for Definitions
Thursday April 25, 2013
|
PERIOD 1 - 8.00 - 9.05 AM PERIOD 2 - 9.05 - 10.10 AM PERIOD 3 - 10.10 - 11.15 AM PERIOD 4 - 11.15 - 12.20 PM
INFORMATION & SUBJECT REGISTRATION DAY (12.20 –1.20 PM)
PERIOD 5 - 1.20 - 2.25 PM PERIOD 6 - 2.25 - 3.30 PM PERIOD 7 – 3.30- 4.45 PM
|
8.3- Definition of hydration, dissociation (p. 383), miscible(p. 384), Like dissolves like (CD example); surfactant (p. 386).
8.5 Solubility and saturation, saturated/unsaturated/super saturation solution (p. 392), solubility curve (p.393).
8.6 Concentration- c=n/v
Standard Solution (p. 401) a
8.7 Preparing Dilution (p. 404, know the dilution equation). p. 405, #1-3.
8.8 Concentration and Consumer Products (p. 411, # 1-6) Do for Blended Learning.
Friday April 26, 2013
Lab Titration Lab on Vinegar
Tuesday April 16, 2013
Calculation Questions- Mole Ratio- 7.1 and 7.2 start of 7.3
Wednesday April 17, 2013
Monday April 8, 2013
There is a briefing for all 2013 OSSLT candidates on Monday, 8th April 2013. The briefing will be held on the 7th floor in the following rooms: KS7.28, KS7.29 and KS7.30 from 10.00-11.00am
Gizmos Sign in www.explorelearning.com
Class code: AHNHJWJZDG
Contamination Remediation Class Activity
OSSLT Test on Friday April 12, 20
Please arrive on main campus at by 7.40 and 7.50. Test will run from 8:00 am to 12.40 pm.
You will have a break at around 10 am for 1/2 hour
Please bring a blue or black pen, HB pencil and eraser and highlighter.
Tuesday April 9, 2013
Calculation Project: Please hand in by Thursday April 11, 2013
Section 6.4, p. 277, #1- 4- Calculating Molar Mass
Wednesday April 10, 2013
Section 6.5, p. 280, Mass and Number of Entities; Tutorial Question,#1 and 2.
Thursday April 11, 2013
Section 6.6: The Composition of Unknown Compounds, p. 286, #1 and 2
Section 6.6 Questions, p. 288# 1-4
Section 6.7: Empirical Formulas, p. 292, # 1
Friday April 12, 2013
OSSLT Test
Monday April 1, 2013
Mentee meeting at 9:00 am in
|
Claire Watson |
Student Activity room (ECA Block) |
Second floor.
Monday March 25, 2013
Please work on the Presentations below
Please finish your "Double Displacement Lab Report" please hand in my Tuesday April 2, 2013
Please watch the "Khan Academy video" to the right.
Hand in the "Assignment for Chemcial Reactions"
Blended Learning Monday April 1, 2013 - April 16, 2013
Work on presentations for Quantities in Chemical Reactions
1. Medical Malpractice because of giving patients too much or too little medication ( how often it occurs and why) Shaheen
2. Chemotheraphy (medical doses and how this chemical compound treats patients) (Fitri choice)
3. Chemical production plants- how they affect soil, air and water supplies. (Jeremy)
4. Agricultural run off in rural places (such as pigmy pigs and other wildlife) (Izzat)
5. Hormones in the waterway system ( birth control and other hormones that are feminizing fishes) (Audrey)
6. Plastic containers in the waterways (Garbage patch in Pacific ocean) (Fitri choice)
7. Radiation therapy ( how it works, the dosage amounts and how often you are given the treatment). (Khalil)
Tuesday April 2, 2013
Review of Lab Gizmos' sheets; review of Environmental and Chemical Processes
Wednesday April 3, 2013
Observation class on Environmental and Chemical Processes
Lecture on Contamination and Chemical Processes
Thursday April 4, 2013
Calculations Mole Box.
Friday April 5, 2013
In Lab Copper on Copper and Mole Lab
Tuesday March 26, 2013
Section 6.4
Start on Mole, p. 268, defintion
Start on Molar Mass, p. 271
Practice Problem, p. 275, #1
Presentations
Wednesday March 27, 2013
Section 6.5, Mass and number of Entitites, p. 280, #1-4
Presentation
Thursday March 28, 2013
Section 6.6, The Composition of Unknown Compounds
Presentation
Friday March 29, 2013
Computer Lab
Monday March 11, 2013
Please take this survey Stop, Start, Continue
Blended learning
Work on Project below (Presentations will be on 13 and 14 of March)
Projects for Chemical Reactions for science, technology, and society/
Chemical Spills- Sheehan
Smelting of elements could be any metals- Khalil
Diamond mining and extraction could include creating jewellery- Sasi
Gas and Oil extraction in Canada- Fitri
Acid Rain- Audrey
Gold extraction mining- Izzat
Tuesday march 5, 2013
In class finish Lab and discuss lab report format.
Wednesday March 12, 2013
Finish 4.2, p. 161, #1-5; Practice problems, p.166, # 2
Sect 4.4, #1-5.
P. 175, practice problems #1.
Section 4.6, #
Link to Slides for 5.3 Elements and Their Oxides
Thursday March 13, 2013
Start of Presentations-
Take home Assignment to be marked after the March break.
Friday March 14, 2013- Start of Presentations and in computer lab for Gizmos lab
Finish Gizmos labs on Limiting Reagents.
Monday March 4, 2012
Choose your next project for Blended Learning:
Work on Project below (Presentations will be on 13 and 14 of March)
Projects for Chemical Reactions for science, technology, and society/
Chemical Spills- Sheehan
Smelting of elements could be any metals- Khalil
Diamond mining and extraction could include creating jewellery- Sasi
Gas and Oil extraction in Canada- Fitri
Acid Rain- Audrey
Gold extraction mining- Izzat
Tuesday and Wednesday March 5 and 6, 2013
Intro to chemical reaction: evidence of chemical reactions, law of conservation of reaction (p. 152) Link to Class Agenda for Chemical Reactions
Intro to Chemical Reaction (4.1), p. 155, Practice #1; Sect Review 4.1, p. 155, # 1-5. Balancing Equations, use of Gizmos.
Wednesday March 6, 2013
Monday Feb 25, 2013
Work on Pendulum Lab to hand in. Please finish your Gizmos Lab. Go to www.explorelearning.com and "Enrol in class" and then put in your "Class Code"
Tuesday Feb 26, 2013
Finish Review Questions and Start Forces
Wednesday Feb 27, 2013
Forces Unit #2, C 3.
Start of Forces (systematic and free body diagrams).
Thursday Feb 28, 2013
Test on Kinematics (Ch. 1 and 2).
Friday March 1, 2013
PD for Teachers and No School for Students
Monday Feb 25, 2012
Due dates: Test on Naming, bonding, periodic table on Web Feb 27, 2013. C 1 and 2 and worksheets. For EXTRA PRACTICE ( p.138, #6-12 MC; #26-28; #29, abc; 32, 38, 39, 40) Check ANSWER------------------------------------>File is chem11_sm
Test on Sections 1-2, 1-3, 1-7, 2-1, 2-2, 2-3, 2.4, 3-3, 3-4, 3-5
Chemistry Feb 25, 2012
Blended learning Review of Unit #1, all students are to attempt the following questions only the Person listed must post their answer to the Moodle Wiki.
Khalil (p. 108, #1-3)
Sasi (P. 87, # 1-7)
Audrey (P.81, #1-4)
Jeremy (P. 73, #1-5ABC)
Shaheen (P.69, #1-2ABC, #3 and 4)
Izzat (p. 115, #1 and 2)
Fitri (p. 41, #1-7)
Tuesday Feb 26, 2013
Review Lab and start balancing equation
Wednesday Feb 27, 2013
Test on Sections 1-2, 1-3, 1-7, 2-1, 2-2, 2-3, 2.4, 3-3, 3-4, 3-5
Thursday Feb 28, 2013
Start on Balancing Equation and Chemical Reaction (4.1)
Friday March, 1, 2013
PD for Teachers and No School for Students
Due dates: Wednesday Feb 20, 2013 Present your G-mail presentation on make-up and fertilizers that you have chosen
Due Dates: Thursday Feb 21, 2013 Hand in Lab questions (from Investigation 3-B making the molecular models) from the Poster project, Questions #1 -4. For question #5 you may use the internet to search for other simple molecules. Type in Google SiO2 and you should get a model from the internet. Or look through your textbook to find other models.
Due dates: Test on Naming, bonding, periodic table on Tuesday Feb 26, 2013. C 1 and 2 and worksheets.
See below for Google doc on Chapt 2.2 Review of Definitions
Monday Feb 18, 2012
Mentorship meeting at 10:00 to 11:30 am at CPU on the 2nd floor
Kpad 2.7. All CPU students must attend class.
Tuesday Feb 19, 2013
Finish naming of transition metals.
Wednesday Feb 20, 2013
Present Presentations everyone must be ready to present.
Thursday Feb 21, 2013
Review for the Test on Tuesday Feb 26, 2013
Friday Feb 22, 2013
Finish lab on activity series, and double displacement reactions.
Monday Feb 4, 2013
Complete the 3 graphs from Chemistry.
Work on Finishing the poster
Introduction of Electronegativity and determining the polarity of molecule. See textbook. Finish poster and models.
Tuesday Feb 5, 2013
Review naming of molecular molecules and IUPAC convention of naming. Introduction of Isotopes and calculations.
Wednesday Feb 6, 2013
Identify the single displacement reactions and activity series model.
Thurs Feb 7, 2013
Quiz on: First 18 elements, name and symbols. Lewis, Bohr- Rutherford diagrams, compare 2 models (ensure you compare modern with initial model) . Calculate Neutrons, protons and electron configuration. Naming ionic compounds.
Friday Feb 8, 2013
Lab on Activity Series, Complete the "Who has the highest Salt tower".
Lab on Properties of Aluminum
Lab on Creating an activity series
Monday January 28, 2013
Please complete the graphs: atomic radii, ionization and electron affinity and also, if you have space "electronegativity".
We will also be using the textbook this week, since people need some time I will post some documents to help you through.
Below is the part of the book we will be using for the first part of explaining the periodic table.

|
openchem11_c01_1_7 (1).pdf Size : 185.603 Kb Type : pdf |
Monday and Tuesday January 29, 2013 we will be working with Ions and naming compounds.

|
openchem11_c01_1_3 (1).pdf Size : 1394.467 Kb Type : pdf |
Monday January 21, 2013
Work on the Periodic Table
Tuesday January 22, 2012
Shortened Periods on Tuesday
Period 1- 8-9 am
Period 2- 9-10 am
Period 3- 10-11 am
Period 4- 11-12 pm
Period 5- 12 -1 pm
Period 6- 1-2 pm
Go to Multi-purpose Hall on Main Campus for a City Survival Talk
2-4 pm.
Review Guide for 11 Chemistry

|
11U CHEMISTRY EXAM REVIEW QUESTIONS June 2010.pdf Size : 223.352 Kb Type : pdf |

|
chemistry practice final exam june 2012.pdf Size : 388.015 Kb Type : pdf |
2nd Practice Exam including full solution key at the end of the exam

|
Practice+Exam.pdf Size : 162.935 Kb Type : pdf |
Monday Nov 19, 2012- NO MORE BLENDED LEARNING- IN class
Finish Percentage Yield and Theoretical Yield questions (last calculation on chapter 7)
Hand in Hydrate Lab send by G-mail (clairetaylors32@gmail.com)
Tuesday Nov 20, 2012- in class finish (7A assignment)
calculations on percentage yield and theoretical yield
concentration calculations
Wednesday Nov 21, 2012- in class finish work on Acid and Bases based on
Titration Labs (the labs where we used the buret)
Thursday Nov 22, 2012- in class work on Acid and Bases
Friday Nov 23, 2012- Calculations quiz (theoretical yield and concentration)
Monday Nov 5, 2012
Mentorship meeting 9:00 am
Tuesday Nov 6, 2012
PERIOD 1 - 8.00 - 9.05 am
PERIOD 2 - 9.05 - 10.10 am
PERIOD 3 - 10.10 - 11.15 am
Assem bly LT2 at Main Campus ( 11.15-12.15 pm)
PERIOD 4 - 12.15 - 1.20 pm
PERIOD 5 - 1.20 - 2.25 pm
PERIOD 6 - 2.25 - 3.30 pm
In class work on assignment 7a, hand in Hydrate lab by gmail
Wednesday Nov 7, 2012 In Lab
Thursday Nov 8, 2012 In class work on solutions
Friday Short Quiz on Names see worksheets
Monday Nov 5, 2012
Mentorship meeting 9:00 am
Tuesday Nov 6, 20
Monday Oct 29, 2012 Finish Hydrate Lab and also Presentations, if you and your partner are completing different topics then just finish your topic.
Tuesday Oct 30, 2012 In Class 8.1 and 8.2 Practice problems and word searches
Wednesday Oct 31, 2012 Lab Titration Lab
Thursday Nov 1, 2012 In class In class start section Ch 9., presentations
Friday Nov 2, 2012 In class presentations
Here are the Solutions to the End of Chapter Revision Questions (Optional),
Chapter 5 p. 194, # 13, 14a, 15, 16a, 18
Chapter 6, p. 230, # 14, 15, and 21.

|
Chapter 5 Answers and 6.pdf Size : 223.077 Kb Type : pdf |
Here are all of the answers to practice problems from Chapter 5

|
Chapter5.pdf Size : 131.161 Kb Type : pdf |
Please hand in your Double displacement reaction lab. You do not need to include photos. A written description will be good enough.
Presentation topics (Solutions and contamination)
Due Dates
Lab Report for 6B Hydrate Lab- 23 October, 2012
Quiz on Moles (see Review Sheet in class) for Friday Oct 19, 2012
Monday October 22, 2012
Presentations will run all week for first 15 minutes keep it within 10 minutes and a 5 minute video - Choose a presentation date running Oct 24, 25, 26, 27 and 28)
You should finish your "Contamination" presentation Tuesday- in class- Calculations of Chemical Equations, using moles Chapter 7.1 Wednesday- IN THE LAB for Neutralization Lab Thursday- In class work on Chemical Equations using moles in Chapter 7.2- Start Presentations from below. Friday- HOLIDAY
Presentation topics ( Present Friday choose by Friday October 12, 2012)
landfill sites (runoff and leachate, Samuel)
agricultual run off
mercury (in the water and sources - JY)
drinking water (bottled- Pacific Garbage Patch- Hamas and Romel)
Water in remote places (Africa/Asia and desert regions)
Changes in water temperature ( what happens to fishes when water is too warm-Ahmed and )
Hormones in the water (birth control pills in water- Amanda)
Air pollution (eating foods locally- Anhu)
Air quality indexes (greenhouse gases- Termeh)
Monday October 15, 2012 work on presentations
Tuesday October 16, 2012- work on Chapter 7
Wednesday October 17, 2012- Shortened periods all students will go to Lakeside- NO LABS-
PERIOD 1 - 8.00 - 8.50 am
PERIOD 2 - 8.50 - 9.40 am
PERIOD 3 - 9.40 - 10.30 am
PERIOD 4 - 10.30 - 11.20 pm
PERIOD 5 - 11.20 - 12.10 pm
PERIOD 6 - 12.10 - 1.00 pm
Thursday October 18, 2012- work on
Friday October 19, 2012- short Quiz on Moles
Monday October 8, 2012
OSSLT workshops from 10-1 pm, all students who have not passed the OSSLT test need to attend workshops in LT1.
Tuesday October 9, 2012
Finish presentations and work on calculations from chapter 6
Wednesday October 10, 2012
In lab, please do not be late.
Thursday finish Moles calculation.
Friday Finish moles work
Monday October 1, 2012
Date: 1st October2012. Time: 9.00 am – 10.00am. Kpad- 2.6
Tuesday- start presentations on Gmail
PERIOD 1 - 8.00 - 9.05 AM
PERIOD 2 - 9.05 - 10.10 AM
PERIOD 3 - 10.10 - 11.15 AM
PERIOD 4 - 11.15 - 12.20 PM
INFORMATION & SUBJECT REGISTRATION DAY
(12.20 –1.20 PM)
PERIOD 5 - 1.20 - 2.25 PM
PERIOD 6 - 2.25 - 3.30 PM
Wednesday- In Lab for 6B determining the chemical formula of a hydrate.
Thursday 6.3
Friday= PD (no class for students but classes for teachers)
Monday Sept 24, 2012 Work on presentations for Friday on gold, minerals, etc, email me your project
INFORMATION & SUBJECT REGISTRATION DAY
Monday Sept 24, 2012 Work on presentations for Friday on gold, minerals, etc, email me your project
Due dates: 4B Double Displacement Lab on Wed 26
G-mail presentation start on Friday 28, some people can start later if you already have spoken to me.
Tuesday Sept 25- Start Chapter 5 on Moles
Wednesday Sept 26- finish lab 4C and start Moles Lab
Thurs Sept 27- work on 5.2 and 5.3, p. 194, #15, 16ab, 17ab, 18, 19, 21
Friday Sept 28- 6.1, defintions of law of definite proportions, mass percent (p. 199); percentage composition (p. 200), practice problem (p. 201), #1; p.204, #5a,b
work on con't start presentations
Monday September 17, 2012
Holiday- enjoy Malaysia day, right Praghi!
Sample Outline for Lab Report Lab Due Wednesday Sept 26, 2012
Gmail Presentation Due on Friday Sept 30- or later for other students- negotiable deadline throughout the week.
Tuesday- Revision of Chapter 4.2, 4.3
Wednesday- Lab Main campus 4-C Copper on Copper
Thursday- Review for Unit test
on TEST from Chapter 3- Determining polar, non polar, Lewis Diagram, show delta (+) and delta (-) of the molecule.
Intermolecular and Intramolecular forces definitions
Naming (polyatomic compounds, ions, transition metals and non metal naming of compounds.
on TEST from Chapter 4-
know the general formulas
know the definitions of single displacement, double displacement, synthesis reaction
balance equations
from the labs determine, which molecules are going to be "soluble" and "insoluble". You must use the tables to determine why a compound is going to give you a precipitate.
Friday- Unit test on naming of chemicals, and chemical reactions (4.1- 4.3). Chapter 3.
Monday September 10, 2012
Memorial for Mr. Michel in MPH at 10:00 am, main campus, on the top floor next to the guard house.
Tuesday in Class- Finish Review Questions
Wednesday in Lab- Double Displacement Reactions
Thursday- in class
Friday- on TEST- Determining polar, non polar, Lewis Diagram, show delta (+) and delta (-) of the molecule, and intermolecular and intramolecular forces and naming (polyatomic compounds, ions, transition metals and non metal naming of compounds.
Friday- do revision 4.1, p. 118, #1, 2, 3, 4 abc, 5.
Practice Problems- p. 122, #11ab, 12 ab, 15 ab, 17.
Blended Learning Sept 3, 2012
Tuesday- In class naming 3. 2 p. 99, # 16, 17; review worksheets. In class project on
Wednesday- In class naming 3.2 p. 84 Section Review 3.2 ; 3.3 Section Review p. 94; 3.4 Section Review #1-6 16, and 17.
Thursday- In class naming 3.4 p. 103, #20, 21, 22, 23, 24 Section Review 3.4.
Chapter 3 Solutions
Choose a topic from the following: batteries, fertilizers, electronic components, PABA, DEET, Zinc, cadium, pulp and paper, platinum, gold, diamonds, and silver.
Friday- In class start chemical equations (c. 4). Finish Review Section.
End of Chapter Review
Review p. 108, # 15, 16.
Review p. 108, p. 17, 18, and 19.
Review p. 108, #23 and #24.
Next Tuesday September 11, 2012
Short Quiz
Model Making, electro-negativity; polar covalent;
definitions of the following: polar covalent, covalent.
Thursday August 30- all presentations must be finished and ready to present. in class.
Monday- Gmail assignment- (PLEASE SEND ME Your PDP document in Gmail when you have finished it- please do by Friday)
Include slides for:
Science (meaning SI units, concept (such as chemical composition meaning what shape or the elements included you may wikipedia this part);
Technology- meaning the machines or devices that are used (such as a machines or medicines that are produced).
Society- meaning how people use the products or science (such as how people fish for sharks but only use the fins, what are some of the society implications).
PLEASE CUT AND PASTE important parts, don't copy whole sections. Make sure to include the links on a page of your Power Point or your G-mail document. Also if you can, find a good video or animation for the class to watch. If you want to create a game you may also . Please make it interesting for all of us. DON'T read slides.
You must either e-mail me or share your gmail document with me:
Gmail address: clairetaylors32@gmail.com
Tuesday August 28, 2012- in class-
Wednesday August 29, 2012- in class
Thursday August 30- all presentations must be finished and ready to present. in class.
Friday HOLIDAY (national day) NO class.
Due dates:
Atom book- August 10, 2012
Unit Test- August 10, 2012 ( Chapter 2 and 1.3 and 1.2). Significant figures and measurement and uncertainty.
Chapter 1 Review, p. 29, # 1, 2, 3, 4, 5, 7 abc, 8, 9 abcde, 10 abc.
Chapter 2 Review, p. 61, # 1, 2, 3, 4, 5, 6 ( on test), 8
Review Sheet PDF
Thursday August 9, 2012 All class will be review. Please finish the questions above.
Monday July 30, 2012
IN class
Tuesday July 31, 2012- in Lab
Below is a link to the experiment that we did today:
http://www.stevespanglerscience.com/experiment/original-mentos-diet-coke-geyser
Wednesday Aug 1, 2012- in class working on review for chapter 2 only
Thursday August 2, 2012- in class
Friday August 3, 2012- in class Quiz on Chapter 2- Periodic Table, names, and groups, ionization, and Ions and atomic radius.The format will be 2 tables, including Bohr-Rutherford and Lewis diagram and calculations of protons, neutrons, and electrons. There are 3 short answer questions.
Shortened Periods on Tuesday Aug 7
TEACHER ADVISOR PROGRAMME
(MENTORSHIP PROGRAMME)
Tuesday 7 AUGUST 2012
(TUESDAY)
Please note the shortened periods on 7 August 2012 to accommodate meeting with students:

PERIOD 1 - 8.00 - 9.00 am
PERIOD 2 - 9.00 - 10.00 am
Meeting with mentors 10.00- 11.30 pm
PERIOD 3 - 11.30- 12.30 pm
PERIOD 4 - 12.30- 1.30 pm
PERIOD 5 - 1.30 - 2.30 pm
PERIOD 6 - 2.30 - 3.30 pm
Monday July 23, 2012
Write the following definitions in your "Atom Book"
X atomic number and atomic mass
IWrite the following Definitions on your "Atom Book":
Definition of 'atomic theory of matter (p. 34 )
Dalton's atomic theory posits 4 rules (p. 35 at the top of the page)
Copy table 2. 1 on p. 35
Describe the physical properties of metals p. 40
Finish the definitions of atomic radius (p. 52) \
Include a blank periodic table (fill out the symbol, name, atomic number and make sure you colour in the columns, such as the alkaline earth metals). ( include 4 rows of the periodic table).
Make sure to include the trends in the periodic table with arrows for ionization energy, electron affinity, and atomic radius. Include a short definition of the above terms.
Include Diagrams of Atom using the following models:
Bohr- Rutherford Model, p. 44, Figure 2.4
Include an example of Lewis diagram, p. 211
IONS from Friday
Include:
ion (diagram and definition), anion, cation, p. 52
Textbook Practice Question
Homework Questions: P. 37, Practice Problem #1;
Try section review: p. 39, # 1 -2.
Tuesday July 24, 2012
Textbook Practice Question
Homework: Practice Problems, P. 22, # 3.
Practice Problems, p. 26, # 4
Section Review P. 24, # 1-6.
Definitions matter p.11
physical and chemical properties p.12
physical and chemical changes p. 25
Include the hand out of the relationship of mixtures and pure substances:
mixture
pure substances
homogeneous mixture
heterogeneous mixture
Wednesday July 25, 2012
In Chemistry Lab 5 on the second floor
Thursday July 26, 2012
In class finishing chapter 2
Friday July 27, 2012
In class finishing chapter 2 starting chapter 3
First Project in Chemistry Due on August 15
Choose from the following topics or choose another one:
Research and show the science, technology and social impacts of the following:
1. fertilizers, pesticides, household cleaning product, materials used in batteries and electronics
2. use of oil paints compared to latex paints
3. flushing pharmaceuticals (such as birth control pills down the drain and the impact on wildlife)
4. addiditves in foods (evaluate the risks and benefits to humans)
5. cosmetics and perfumes
6. cleaning products such as mildew remover
Remember to include:
Science- such as showing the science part of the project such as the chemical composition
Technology- how we use the products such as the machines
Society - how humans use the products for good purposes or not so good purposes

|
OSSLT+Strategy+Notes+2010+Version+2.pdf Size : 1020.238 Kb Type : pdf |
In class this week.
Tuesday May 22, 2012
In chemistry Lab 2
Monday Review Sheet pH review and cross word
Titration lab p. 402, Lab: Investigation 10-B
Do, p. 398, Practice Problems 12 and 13.
Blended Learning on Monday May 14, 2012
Finish These problems for Tuesday May 15, 2012. A quiz on Thursday will be on these questions and the laws.
Boyle’s Law, p. 434, #2, 3
Charles Law , p. 446, #9 and 10
Gay Lussac’s Law, p. 448, #13 and 15
Combined Gas Law, p. 456, #17, 18 and 19
Ideal Gas Law- too many people were away so you must read p. 472-488 by Tuesday May 15, 2012
Tuesday May 15- In class finishing Ideal Gas Laws
Wednesday May 16- Review for Quiz on Thursday, p. 305, #1, 2,
p. 446, # 5a, #11
p. 457, #20
p. 461, # 23,
Thursday May 17- Quiz on Gas Laws, Section 11.1- 11.2-11.3 and 11.4 and 12.1
Friday Review of Solutions and Titration
Blended Learning: Monday May 7, 2012
Monday May 7- finish the Hydrate Lab (this must be typed)Send me the lab report by Gmail, either complete the lab report in Word on in Google Docs
Tuesday May 8- in class no Labs for 3 weeks.
Blended Learning: Monday April 23, 2012
Monday April 23- finish the Hydrate Lab (this must be typed)Send me the lab report by Gmail, either complete the lab report in Word on in Google Docs
; finish the other two labs the (7A Limiting Reactions and Chalk labs) you do not have to do these labs on the computer, just write the answers to the questions.
Tuesday: Lab on 9A- Lab
Wednesday: In Class finish Definitions Chapter 8. P.286, Aqueous solution, miscible, immiscible, alloys, solubility, saturated solution, unsaturated solution.
Thursday: Continue C. 8 - Solutions Start working on this
|
CPU INFORMATION & SUBJECT REGISTRATION DAY 26 APRIL 2012 (THURSDAY) 12.20 PM – 1.20 PM
|
Please note the shortened periods on 26 April 2012 to accommodate Information & Subject Registration Day activities:
|
PERIOD 1 - 8.00 - 9.05 AM PERIOD 2 - 9.05 - 10.10 AM PERIOD 3 - 10.10 - 11.15 AM PERIOD 4 - 11.15 - 12.20 PM
INFORMATION & SUBJECT REGISTRATION DAY (12.20 –1.20 PM)
PERIOD 5 - 1.20 - 2.25 PM PERIOD 6 - 2.25 - 3.30 PM PERIOD 7 – 3.30- 4.45 PM
|
Friday: Start C. 9:
Chapter 8 and 9 Review
Friday April 27, 2012
Draw Figure 8.7, p. 292, on your poster, include the concept map of Polar and Non-Polar Compounds.
Write out the definition (p. 292) of dipole, dipole-dipole attraction, hydrogen bonding, ion-dipole attraction. On page 294 write out non-electrolytes, Write out table of Solubility (Figure 8.2) p.295.
On p. 306 include a few of the items on Table 8.3 ( you can include only the items that you recognize, for example you may know what brass is but do not know what duralumin is).
Include the Concept Organizer on p. 318, on your poster as well.
From 9.1 in your textbook include the Chem Fact table at the bottom of p.330 on your poster as well.
Include Figure 9.2 A and 9.2 B on your poster as well.
Include Figures and table on p. 334 on your poster as well. Include Figure 9.6 and Table 9.3 and 9.4.
Blended Learning: Monday April 16, 2012
Monday April 16- finish the Hydrate Lab
Send me the lab report by Gmail, either complete the lab report in Word on in Google Docs
Finish Chapter 7 Questions: p. 244, Questions # 11, 12, 13 and 14. Answers ARE ON THE RIGHT
Tuesday: Lab on 7A- Lab
Wednesday: In Class finishing Chapter 7. P.254 23, 24,p.257 27. Questions Percentage yield p. 262, questions 31, 32, 33, 34
Thursday: Continue C. 8 - Solutions Start working on this
Friday: Short Quiz on Chapter 7, C 7.1, 7.2, and 7.3. 5 Calculation Questions
Based on Questions From Last Week.
Blended Learning: Monday April 9, 2012
Mentee Meeting on Monday: in KPad - 2.6 (Please bring a labtop or you will go to the computer lab to finish your PDP)
Tuesday: Lab on 6B- hydrate- you must do a lab report on this lab so make sure you come to class.
Wednesday: holiday
Thursday: Continue C. 7 - Finish presentations and hand in Chapter 6 Assessment Questions
Friday: Continue C. 7
2nd last sections: Solutions- C. 8 very important, C9.1 to 9.3- C.10.1 and 10.2
Last Section: Gases (C. 11 and 12)
A preview for Grade 12- Organic Chemistry
Blended Learning: Monday April 2, 2012
Work on your Presentation for Friday. Remember you need to relate the following:
Science- at least 3 slides explaining science concepts.
Technology- how your science concept is translated into machinery or equipment.
Society- how people use the different science concept and how it affects their lives.
Tuesday April 3, 2012
In class, no lab sessions. Please bring your textbook.
Blended Learning on Monday March 26, 2012
Please revised from this website:
http://www.eqao.com/Students/Secondary/10/10.aspx?Lang=E&gr=10
Blended Learning for Monday Mar, 12, 2012
Mentor meeting at 10-11 in KPad 2.6 with PDP in hand
http://www.explorelearning.com
Use the class code to set up account
U9VMFWXVPQ
Blended Learning for Monday Mar 5, 2012
Decomposition Reactions (Do p. 123, # 14-15)
Combustion Reaction ( Do p. 124, #17, 18, 19, 20)
Do Section Review (P.125, #1-6)
Activity Series Reactions (Like the Lab with the metals) P. 131, # 22
Single Displacement Reactions (p. 131, # 23, 24, 25)
Decomposition Reactions (Do p. 123, # 14-15)
Combustion Reaction ( Do p. 124, #17, 18, 19, 20)
Do Section Review (P.125, #1-6)
Activity Series Reactions (Like the Lab with the metals) P. 131, # 22
Single Displacement Reactions (p. 131, # 23, 24, 25)
Blended Learning for Monday Feb 27, 2012
Finish the lab questions from the first Lab (Activity Series)- This lab can betyped or hand written. (4A Lab Creating an activity series- p.128)
Finish the lab questions from the Second Lab ( Double Displacement reaction) This lab must be typed please send me what you have in terms of the table to my Gmail account ( 4B Observing Double displacement Reaction- p. 136). clairetaylors32@gmail.com
Tuesday Feb 28, 2012 in Chemistry Lab # 5
Wednesday Feb 29, 2012- Reactions Balancing Equations (Do, p. 116 # 5, 6, 7, 8) Section Review 4.1 ( Do, p. 118, 1-5)
Thursday and probably to Monday Mar 4, 2012
Synthesis Reactions ( Do p. 122 # 10-13)
Decomposition Reactions (Do p. 123, # 14-15)
Combustion Reaction ( Do p. 124, #17, 18, 19, 20)
Do Section Review (P.125, #1-6)
Activity Series Reactions (Like the Lab with the metals) P. 131, # 22
Single Displacement Reactions (p. 131, # 23, 24, 25)
Blended Learning Monday Feb 20, 2012
Create a Review Sheet (A4 size paper) - must be hand written, no typing:
Tuesday February 21, 2012 go to Chemistry Lab 5
Test on Ch. 2 and 3 ( Friday February 24, 2012)
Please make a Facebook comment.
Definition
Ionization
Atomic Radii
Electron Affinity
Electronegativity
Periodic Table names and symbols for the first 18 elements
Review Questions (Chemistry 11- Textbook) p. 107, # 1-7,
p. 108, # 16-19
Weed of Monday Feb 20, 2012
Check my Website for Blended Learning assignment:
www.preuniversitycourses.com
Tuesday Feb 21, 2012
In the Lab: Double Displacement Reaction
Wednesday Feb 22, 2012
Finish Review for test (Ionic compounds, Covalent compounds, ionization energies, atomic radii) Periodic Table
Communication Questions - You must review your ractions from Lab on Tuesday Feb 14, 2012
Thursday Feb 23, 2012
Start Chemical Naming and Reactions
Friday Feb 24, 2012
Test on Ionization, Chapters 2 and 3
EXAM Revision
Check your Chapter 7 Review Questions. p. 271, # 1,2,4 5, 7, 11. Here are the full solutions. Full solutions
This section deals with explaining the effects of changes in temperature and the affect of solubility of oxygen in lake water. Explain what happens to the wildlife.
Watch the video. Determine what is important about learning about dissolved nutrients in an ecosystem. What are the detrimental effects on the environment.
This video corresponds to p. 298 in your textbook
Examples of passive voice in lab reports
Taken from: http://guides.lib.purdue.edu/c.php?g=352816&p=2377936
Correct:
200mL of distilled water was poured into a 500 mL beaker.
Incorrect:
I poured 200mL of distilled water in a beaker. (active voice)
Pour 200mL water in a beaker. (direction/command)
Correct:
The covered crucible was mounted on a ring stand.
Incorrect:
We put the crucible on a ring stand. (active voice)
Set the crucible on a ring stand. (direction/command)
Correct:
The temperature was initially measured at 75°C.
Incorrect:
I measured the temperature at 75°C. (active voice)
Measure and write down the temperature. (direction/command)
Chapter 7: Stoichiometry in Chemical Reaction (combining moles and chemical reaction)
7.1 Mole Ratios in Chemical Equations;
Do: Solving mole ratio problems, p. 319, #1-3
Do: 7.1 Questions, p. 320, #1-5. Answers to 7.1
7.2 Mass Relationships in chemical Equations,
Do: p. 321, definition of stoichiometry, p. 323, #1-3
Do: Questions 7.2, p. 1-5.
7.3. Which Reagent Runs out First? (limiting reagent)
Do: Definitions of: limiting reagent; excess reagent ( p. 3260)
Do: Questions p.330, #1-3
7.4 Calculations Involving Limiting Reagents,
Do: p. 332, Practice, #1-3
Do : P. 334, Practice, #1 and 2.
Do: 7.4 Questions, #1-4
7.5 Actual/ Theoretical and Percentage Yield
Do Practice Question, p.338, #1and 2
Do Questions P.338, #1-5, 5,6,7
End of Chapter Review
Grade 11- Read 6.3-
Define mole (p. 268); Avogadro's constant (p. 268);
Do practice problems, p.270; #1
Do 6.3 questions, p. 270, #1-6
Read 6.4
Define molar mass (p. 271)
Do practice p. 275, #1
Below is the soltion Key

|
chem11_sm_u1_r.pdf Size : 536.652 Kb Type : pdf |
Solutions of Section Review Questions (3.1 to 3.4)

|
review answers for chapter 3.pdf Size : 452.029 Kb Type : pdf |

|
Chapter3.pdf Size : 155.401 Kb Type : pdf |

|
Chapter4yola.pdf Size : 122.419 Kb Type : pdf |
Relevant PDF files below are the solutions to all of the balanced equation questions.

|
Chapter8.pdf Size : 95.61 Kb Type : pdf |

|
Periodic Table.pdf Size : 41.201 Kb Type : pdf |
Here is the PDF original file, it is easier to read the smaller values. You may want to print off a colour copy to help you.

|
Chapter6.pdf Size : 123.532 Kb Type : pdf |

|
Chapter7.pdf Size : 250.619 Kb Type : pdf |
Sample Student Presentation on Gold

|
gold.pdf Size : 147.587 Kb Type : pdf |
Solubility Curves.
On p. 290, determine the definition of solubility.
Is the amount of __solute_______ that dissolves in a given volume of solvent at a certain temperature.
Review the definitions below for the exam.
solution
solvent
solute
dissolve
Here are Solubility Curves below: In your notebook write out which substance decreases in solubility as the temperature increases.
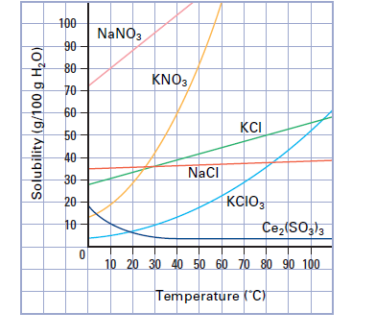
Here are some EXAM Questions:
The graph above shows the solubility of various substances plotted against the temperature of the solution.
a. Which substance decreases in solubility as the temperature increases?
b. Which substance is least soluble at room temperature? Which substance is most soluble at room temperature?
c. The solubility of which substance is least affected by a change in temperature?
d. At what temperature is the solubility of postassium chlorate equal to
40 g/100 g of water.
e. 20 mL of a saturated solution nitrate at 50 degrees C i scoller at
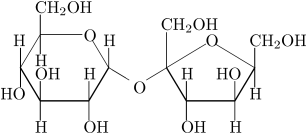
Non electrolytes as below. In the diagram below write out where the water molecules would attach it self to this molecule. Hint p. 294 in your textbook.
You built the model below in class. Review for the exam questions
ANSWERS TO EXAM QUESTIONS: